Explain the Difference Between Osmosis and Diffusion in Cells
Diffusion is the process by which particles movie from high concentration gradient to lower concentration. Movement or transportation in this process of osmosis tends to equalize the concentration of the solvent which doesnt occur.

Major Difference Between Osmosis And Diffusion Similarities Yb Study
Moreover the goal of diffusion is to attain equilibrium in the energy concentration.

. The primary differentiating factor between the two systems is the medium in which they are employed. It helps in the uptake of minerals and nutrients. Furthermore osmosis requires a semi-permeable membrane while diffusion does not.
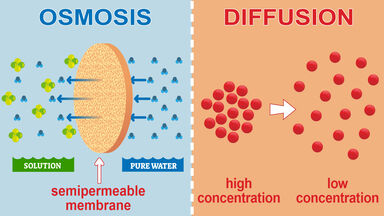
Osmosis can take place only through a semi-permeable membrane. Movement or transportation in diffusion tends to equalize the concentration throughout. Diffusion is the movement of molecules such as oxygen in and out of a cell.
On one hand diffusion happens in any medium be it solid liquid or gas whereas osmosis can only happen in a liquid environment. Summary of difference between Osmosis and Diffusion. The process by which water molecules are able to diffuse through the cell membrane.
Substances move from a high to a low concentration down a concentration gradient. In diffusion both solvent and solute molecules can diffuse. Fusion may refer to a lot of things.
This movement could occur by. Osmosis is the movement of water molecules through the cell. Osmosis depends upon the number of the solute particles which are dissolved into the solvent.
One big difference between osmosis and diffusion is that both solvent and solute particles are free to move in diffusion but when we talk about osmosis only the solvent molecules water molecules cross the membrane. Osmosis happens across a partially permeable membrane while diffusion does not need a membrane it happens directly in the fluid. In biology diffusion is only discussed in terms of the movement of solutes across the cell membrane through intracellular fluid or through extracellular fluid.
The main difference between osmosis and diffusion is that osmosis requires a semi permeable membrane. Osmosis can occur only in liquid medium. Here are the more common things that fusion refers to.
Diffusion Does not depend on solute potential pressure potential or water potential. The difference between osmosis and diffusion is that diffusion does not involve a semi permeable membrane. Other solvents are not considered.
In Osmosis the particles of solvent molecules travel across the semipermeable membrane while in diffusion the molecules from a higher to lower concentration. Diffusion is the movement of molecules such as oxygen in and out of a cell. The concentration of the solvent does not.
Osmosis is the diffusion of solvent mostly water through a semipermeable membrane down its concentration gradient. One big difference between osmosis and diffusion is that both solvent and solute particles are free to move in. Without the need for energy or actively ie.
Osmosis does not helps in the uptake of minerals and nutrients. Explain the difference between osmosis and diffusion in cells. The process by which water molecules are able to diffuse through the cell membrane.
The diagram compares diffusion of sugar molecules and osmosis. The movement of materials always occur in cells. Osmosis is the movement of water molecules but diffusion is for any molecule.
This can be confusing to understand because while the solvent particles are moving from higher to lower solvent. Food materials and also remove waste products eg. Osmosis is the net movement of water H_2O molecules from a region of less negative water potential to a region of more negative water potential down a water potential gradient through a membrane.
Filtration is a process of transportation of substance passively between the compartments. Carbon dioxide oxygen water food substances wastes eg urea. Aside from those said earlier one of the major differences between the two is the type of medium where these processes happen.
Osmosis is the movement of water molecules through the cell. These substances may pass through the membrane passively ie. Osmosis is a special version of diffusion in which water molecules move from a higher water potential to a lower water potential across a semipermeable membrane.
The movement of small molecules from a higher concentration to an area of lower concentration. There are two passive processes which can occur in cells. Diffusion can occur between any medium such as gas to gas liquid to liquid liquid to gas solid to gas and solid to liquid.
Osmosis can only function in a liquid medium but diffusion can occur in all three mediums solid liquid and gas. Include in your answer which type of the molecule is moving for each process in which direction the molecules in question are moving low to high or high to low concentrations. Cells need to transport useful substances eg.
Diffusion can occur with and without a membrane. During osmosis cells undergo different states which reflect the net movement of water molecules. In biology osmosis is only discussed in terms of the movement of water through the cell membrane.
Explain the major difference between diffusion and osmosis. This occurs naturally because the system seeks balance or equilibrium.
Main Difference Between Osmosis And Diffusion In Biology

No comments for "Explain the Difference Between Osmosis and Diffusion in Cells"
Post a Comment